Next Generation Antimicrobial Platform Focused on Wound Healing, Disinfecting and Decontamination
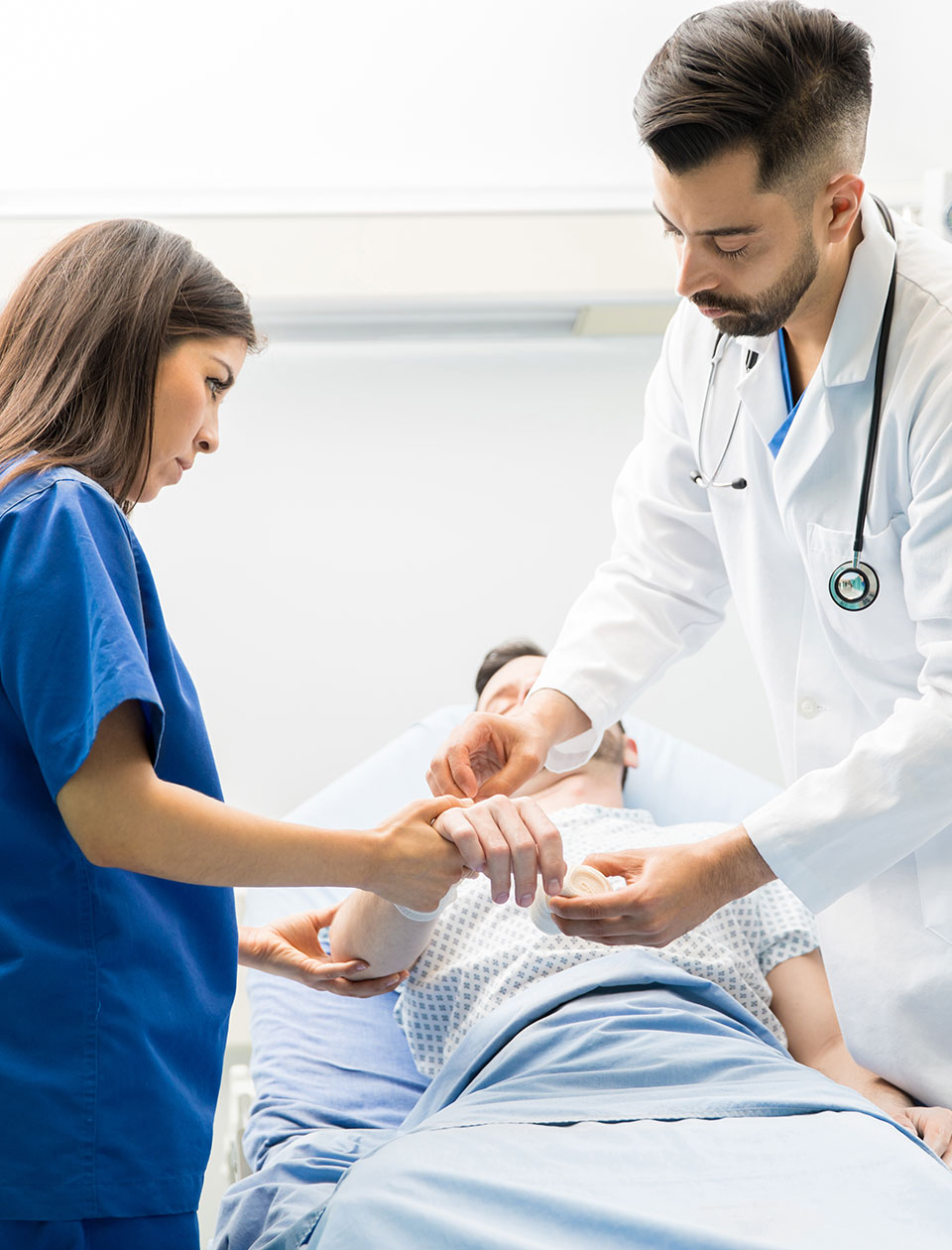
The Problem
Antimicrobial Resistance (AMR) Pathogens is a Global Healthcare Crisis
Currently, antimicrobials are used to prevent and treat infections. AMR occurs when these medicines no longer work, making infections difficult to treat, resulting in increased risk of disease and death. Today, AMR causes 1+ million deaths annually, and AMR deaths are projected to grow by 70% to 39M+ by 2050.
Armis Biopharma’s Veriox® technology platform targets these AMR pathogens.
Our Mission
Our mission is to decrease the quantity and severity of infections that cause human suffering and death, through the creation and development of novel, targeted, antimicrobial therapies
Armis Biopharma® Technology
The highly selective action of Peracid – Hydrogen Peroxide has been independently verified by government and private laboratories to eliminate a huge number of bacterial, viral, fungal and toxic agent threats present in today’s environment.
Veriox® features a novel mechanism of action that reduces the likelihood of contributing to resistance against Gram+, Gram-, viral, fungal pathogens and destroys chemical/biological warfare agents.
High Concentration of Active Agent with Low Systemic Exposure

Versatile Platforms in the War on Resistant and Preventable Infection
Contact Us to Learn More
Have a question? We’re happy to help! Please fill out the form and we will get in touch with you shortly.
